Welcome from EUS-AAEM
The Emergency Ultrasound Section of the American Academy of Emergency Medicine (EUS-AAEM) was founded to foster the professional development of its members and to educate them regarding point-of-care ultrasound. This group will serve as a venue for collaboration among medical students, residents and practitioners who are interested in point-of-care ultrasound. The purpose of our group is to augment the knowledge and expertise of all emergency medicine specialists and to advocate for patient safety and quality care by endorsing bedside ultrasound. Membership is not limited to fellowship trained physicians. All emergency medicine practitioners passionate about ultrasound are welcome to join and participate.
We are proud to publish our e-newsletter with original contributions from many of our members. We encourage all members to submit for future editions. Topics include but are not limited to educational, community focus, interesting cases, resident and student section, and adventures abroad.
For more information, visit our webpage.
In this Issue
- President’s Message
- Building Blocks
- Fellow Section
- POCUS Saves
- Procedure Section
- EUS-AAEM Poster Competition Winners from AAEM24
President’s Message
Hello Fellow EUS-AAEM Section Members!
Welcome to the Winter Edition of the POCUS Report Newsletter. The goal of our section this year is to reconnect with our members. We want members to be included in our section meetings and to give us ideas on how we can address our section as a whole. If you are interested in helping in our section please reach out to our staff liaison Roxanne Dobbs, and she will direct you towards whichever project you are interested in.
As a reminder we currently have our UnMute Your Probe series running. This year will be slightly different with some podcast format sessions and new topics. Check out the schedule Unmute Your Probe – AAEM.
Additionally, we are looking forward to a fun AAEM25 in Miami and have already started planning events.
For this edition of the POCUS Report we are happy to include contributions from our members including the winners of the AAEM24 Poster Competition. We have included articles of interest to all levels of ultrasound skill and some helpful information on US procedural guidance.
Happy Reading!!
Alexis Salerno, MD FAAEM FPD-AEMUS
Chair EUS-AAEM Section
alexis.salerno@som.umaryland.edu
Building Blocks
Diagnosis of Central Retinal Arterial Occlusion on Point of Care Ultrasound
Karalee Bluhm, MD; Danielle Biggs, MD, FAAEM, AEMUS-FPD; Mary Rometti, MD; Jeffrey Greco, MD, AEMUS-FPD; and Travis Masood, MD
Case
A 74-year-old female presented to the Emergency Department (ED) with a sudden onset of painless vision loss in her right eye one hour prior to arrival. She was in the casino playing slot machines when she suddenly lost vision in her right eye. No prior similar episodes or strokes. She denied headaches, fevers, eye pain, or other complaints. A Code Stroke was activated and the patient was taken directly to computed tomography (CT). Pending CT results, a bedside ocular ultrasound was performed of her right eye, which revealed a hyperechoic retrobulbar lesion in her right eye consistent with a clot. Ophthalmology and Neurology were consulted. The patient was placed on dual antiplatelet therapy. CT angiography of her brain and neck revealed severe stenosis of her right internal carotid artery. Vascular surgery was consulted and the patient was scheduled for a right endarterectomy.
Discussion
Frequently, patients present to the Emergency Department (ED) for painless vision loss with ocular emergencies accounting for about 3% of all ED visits.1,2 Point-of-care ultrasound (POCUS) has positively impacted the way ocular emergencies are diagnosed and treated in the ED by decreasing time to diagnosis and making the diagnosis more accurately than compared to the previous gold standard.1,3,4 Though POCUS can identify retinal detachments, vitreous hemorrhages, and posterior vitreous detachment, it can also be helpful in the evaluation of a central retinal artery occlusion (CRAO).1,4 Using POCUS to aid in the diagnosis of a CRAO is efficient and enables ED clinicians to start mobilizing resources to initiate treatment and disposition for the patient.3
Though a CRAO is an infrequent presentation in the ED, the timely identification of a CRAO is vital because the occlusion of the central retinal artery creates vision loss.1,2,3 Classically, patients will present with sudden onset, painless, monocular vision loss.4,5 CRAOs happen when there is a partial or complete occlusion of the central retinal artery, resulting from an embolic or non-embolic physiology.1 CRAO occurs most commonly from a clot that embolizes to the retinal artery.5 Within 90-100 minutes, the retina starts developing irreversible damage.4 About 80% of patients with CRAOs can have an ultimate visual acuity of 20/400 or worse.1
On an ocular ultrasound (US), visualizing a hyperechoic area in the optic nerve sheath next to the retina is known as the retrobulbar ‘spot sign’ (RBSS).4 Smith et al cites a few smaller studies which report that visualizing a retrobulbar spot sign is 100% specific and 31% to 83% sensitive for the presence of a thromboembolic CRAO. Traditionally, a dilated fundoscopic exam is the gold standard for evaluation of sudden onset painless vision loss, but many ED physicians are not routinely comfortable with performing one or may be limited by resources.2,4 Furthermore, ocular US has been found to be more accurate than a dilated fundoscopic exam.1
Conclusion
In summary, point-of-care ultrasound is a preferred tool for diagnosing CRAO in the Emergency Department due to its speed and effectiveness. While fundoscopy is the traditional method, POCUS offers rapid, non-invasive imaging that can quickly identify key signs of CRAO, such as the retrobulbar ‘spot sign.’ This is particularly valuable in emergency settings where time is critical, and fundoscopy may be difficult or less accurate. POCUS allows for immediate diagnosis and intervention, improving patient outcomes in cases of sudden, painless vision loss.
References
- Smith AT, Wilbert CD, Ferre RM. Using the retrobulbar spot sign to assist in diagnosis and management of central retinal artery occlusions. Journal of Ultrasound in Medicine. 2019; 39(1): 197-202. PMID: 31228289. DOI: 10.1002/jum.15073.
- Rosen P (ed). Emergency Medicine Concepts and CLinical Practice, ed 4. St. Louis: Mosby, 1997, pp 2243-5
- Tanaka HL, Popa D, Hayden SR. Diagnosing central retinal artery occlusion via point-of-care ultrasound in the emergency department. The Journal of Emergency Medicine. 2021; 60(5): 655-658. PMID: 33579659. DOI: 10.1016/j.jemermed.2020.12.003.
- Taylor GM, Evans D, Doggette RP, et al. Painless loss of vision: rapid diagnosis of a central retinal artery occlusion utilizing point-of-care ultrasound. Oxford Medical Case Reports. 2021; 6: 209-212. PMID: 34158954. DOI: 10.1093/omcr/omab038.
- Simon A, Gibbons R, Costantino T. Point of care ultrasound assessment of acute monocular vision loss. Visual Journal of Emergency Medicine. 2018; 13: 48-49. DOI: 10.1016/j.visj.2018.06.006.
- Varma DD, Cugati S, Lee AW, Chen CS. A review of central retinal artery occlusion: clinical presentation and management. Eye (Lond). 2013 Jun;27(6):688-97. doi: 10.1038/eye.2013.25. Epub 2013 Mar 8. PMID: 23470793; PMCID: PMC3682348.
The Importance of Utilizing a Systematic Approach When Evaluating for Lung Pathology with Ultrasound
Kevin Mancheno, MD PGY-II and Gabriela Rivera-Camacho, MD
Case
A 50-year-old woman with no past medical history presented to the emergency department (ED) with shortness of breath. This was the patient’s second visit in the last two days. During her initial visit, the patient presented with sudden onset shortness of breath and pleuritic left sided chest pain radiating to the left neck which occurred while seated at her work. During this initial visit the patient was diagnosed via x-ray with a small simple spontaneous left-sided pneumothorax with 1.3 cm of pleural separation and was admitted for overnight observation and serial x-rays. The patient left against medical advice and subsequently followed up with her PCP the next day. The PCP ordered a chest x-ray showing the pneumothorax had grown from 1.3 cm to 1.8 cm and the patient was instructed to return to the ED. Her vital signs were normal and her physical exam was remarkable for decreased breath sounds on the left side and absence of tracheal deviation. Bedside thoracic point-of-care ultrasound (POCUS) at the left 2nd intercostal space (ICS) revealed an area of lung sliding with a transition point to absent lung sliding consistent with a lung point (fig. 1). Further scanning at the left 3rd ICS identified two transition points consistent with a double lung point (fig. 2). To further delineate the lung point, a lower ICS segment of the lung was scanned as well, this one, revealing normal sliding (fig. 3).
Figure 1: Lung point identified on the left 2nd ICS. M-mode shows a “barcode” sign consistent with pneumothorax.
Figure 2
Figure 3: Caudal scan of the same lung showing normal lung sliding. In M-mode, this is consistent with the “seashore sign” where the pleural line takes on a sandy appearance due to motion of the lung against it. This is lost in a pneumothorax.
Repeat upright chest x-ray in the ED confirmed that the pneumothorax had increased in size from an intrapleural distance of 1.3 cm to 1.9 cm. Since she remained with normal vital signs, she was admitted for oxygen therapy in consultation with trauma surgery for consideration of thoracostomy if clinical worsening occurred. Trauma surgery recommended conservative management with supplemental oxygen, pain control, and serial examinations with plan for repeat chest x-ray the following morning. The following day, CXR imaging showed the pneumothorax had decreased in size and the patient was subsequently discharged home with instruction for outpatient follow-up and strict return precautions.
Discussion
POCUS has been established as an important modality for the diagnosis of pneumothorax, especially with its ability to provide rapid real-time images at the bedside without the delay that comes with radiographs. In the past, portable chest radiography was considered the optimal means of identifying pneumothorax at the bedside. However, a 2014 systematic review noted the sensitivity and specificity of pneumothorax on ultrasonography to be 0.87 and 0.99, respectively (1). Similarly, a systematic review by Wilkerson et al (2010) showed the sensitivity and specificity for thoracic POCUS was 86-95% and 97-100% respectively, compared to chest x-ray which showed a sensitivity and specificity of 28-75% and 100% respectively (2). This highlights that an adequately done thoracic POCUS is more accurate at ruling out pneumothorax than a chest radiograph.
Given the positionally dependent nature of a pneumothorax, lack of systematic scanning technique of all intercostal spaces can lead to missed diagnosis and potential catastrophic complications. In an upright patient we expect to see a pneumothorax at the apices versus a supine patient where we would expect to see the same over the anterior thoracic cavity.
The BLUE protocol is an algorithmic approach designed to assist the user in determining the cause for dyspnea in the undifferentiated patient and involves dividing and scanning the thorax in three places: 2 on the anterior chest wall (upper & lower BLUE point) and 1 located semi posteriorly (posterolateral alveolar and/or pleural syndrome [PLAPS] point) (3, 4). This protocol is useful to evaluate for pathology such as pulmonary edema, pleural effusions, consolidation, and pneumothorax. Emergency physicians (EP) often perform a thoracic ultrasound to evaluate for pneumothorax as part of the Extended Focused Assessment with Sonography in Trauma (EFAST). Operators often evaluate one or two ICS in each hemithorax. It is important for EPs to remember that evaluating multiple anterior ICSs can improve sensitivity when evaluating for pneumothorax.
The lung-point is a transition point where lung sliding from the expanded lung is seen at the same ICS as absent lung sliding from the collapsed lung. A lung point is 100% specific and has 100% positive predictive value for pneumothorax, with a sensitivity of 67%. (5) In our case, the patient had a lung point in the 2nd ICS and a double lung point at the 3rd ICS. If this were a first-time presentation in a supine patient and the operator had only performed an evaluation of one ICS, this pneumothorax could have been potentially missed. This highlights the importance of performing a systematic and complete evaluation of each hemithorax starting at the clavicle at the mid-axillary line evaluating each ICS caudally until the diaphragm is visualized in order to avoid missing pneumothorax.
References
- Ebrahimi A., Yousefifard M., Kazemi H. Met al., Diagnostic accuracy of chest ultrasonography versus chest radiography for identification of pneumothorax: a systematic review and meta-analysis, Tanaffos. (2014) 13, no. 4.
- Wilkerson RG et al. Sensitivity of Bedside Ultrasound and Supine Anteroposterior chest Radiographs for the Identification of Pneumothorax After Blunt Trauma. Acad Emerg Med 2010. PMID: 20078434
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care 4, 1 (2014). https://doi.org/10.1186/2110-5820-4-1
- Bekgoz B, Kilicaslan I, Bildik F, Keles A, Demircan A, Hakoglu O, Coskun G, Demir HA. BLUE protocol ultrasonography in Emergency Department patients presenting with acute dyspnea. Am J Emerg Med. 2019 Nov;37(11):2020-2027.
- Lichtenstein D, Mezière G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000 Oct;26(10):1434-40. doi: 10.1007/s001340000627. PMID: 11126253.
- Taylor A, Anjum F, O’Rourke MC. Thoracic and Lung Ultrasound. [Updated 2023 Apr 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
Fellow Section
POCUS of a Testicular Rupture
Zachary Ginsberg, MD and Gabriela Rivera-Camacho, MD
Case
A 49-year-old man with no significant past medical history presented to the Emergency Department with a testicular injury. He was hit in the testicles with a baseball the day prior and had constant right testicular pain since the injury with associated bruising and swelling of the right testicle.
He presented with normal vital signs. On physical exam, the right hemiscrotum was noted to have significant ecchymoses and edema. There was tenderness to palpation of the right testicle. On point-of-care ultrasound, the right testicle appeared enlarged with a heterogeneous echotexture and abnormal contour (Figure 1). The epididymis was enlarged. A large hematocele was present with disruption of the tunica albuginea (Figure 2).
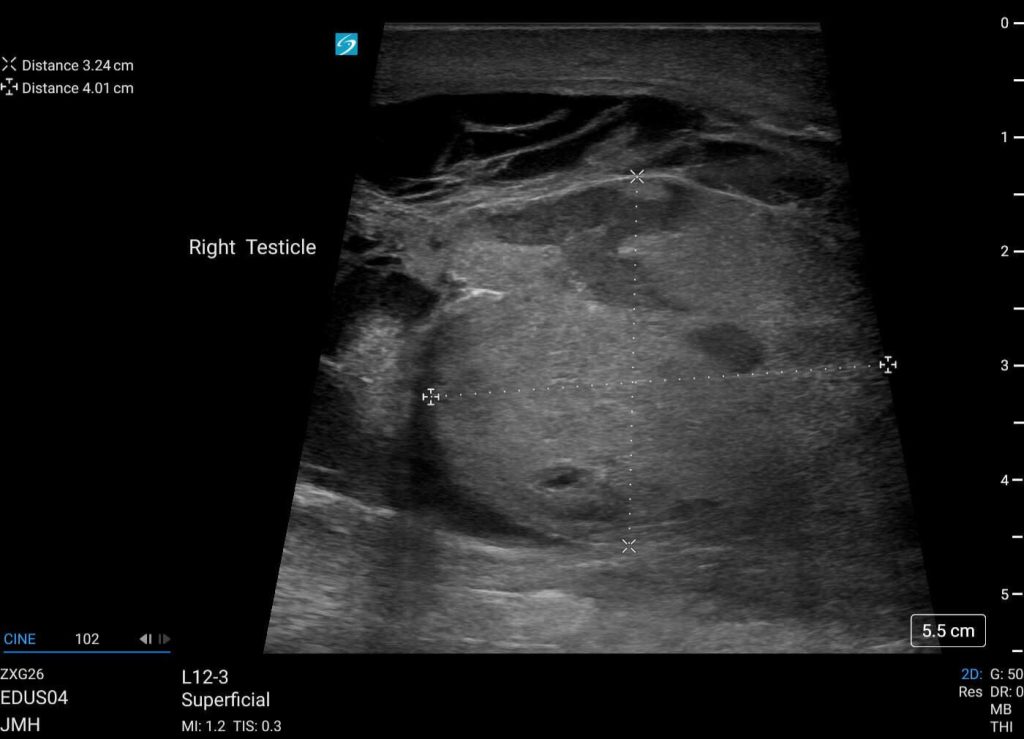
Figure 1: Right testicle appears enlarged with a heterogeneous echotexture and abnormal contour.
Figure 2: In the right testicle, a large hematoma is seen with disruption of the tunica albuginea.
Color doppler was present suggesting preserved blood flow throughout most of the testicle (Figure 3). This was concerning for testicular rupture. The left testicle appeared normal with no hematocele, intact tunica albuginea, preserved vascular flow, and normal in size and homogeneous parenchyma (Figure 4, 5).
Figure 3. Color Doppler shows vascular flow throughout most of the right testicular parenchyma.
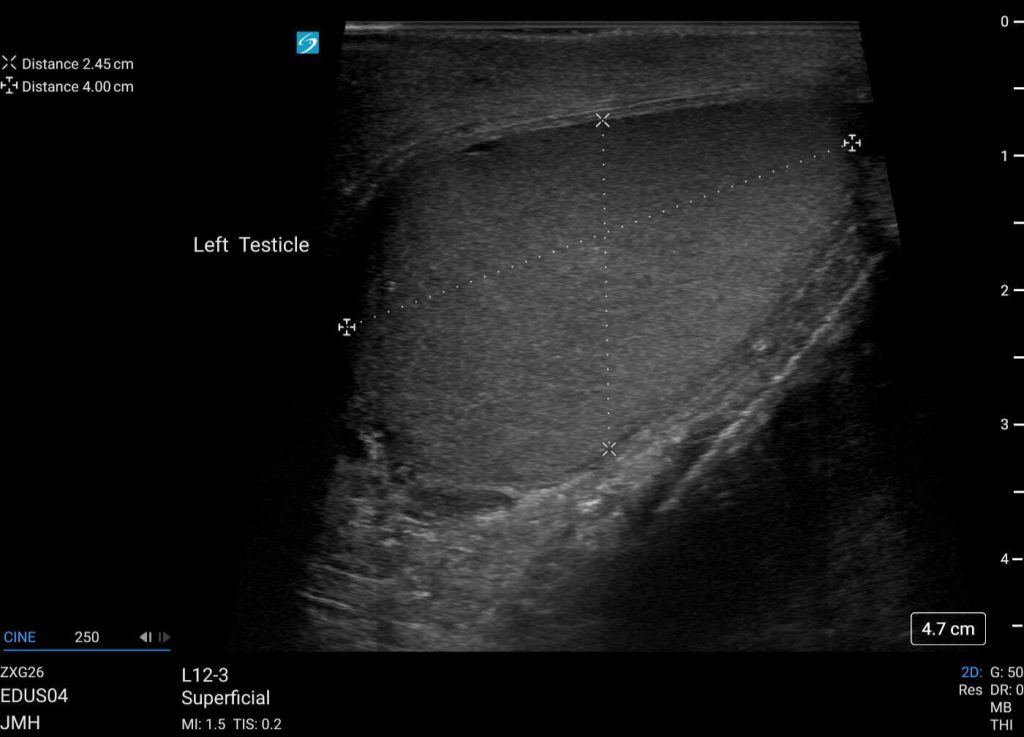
Figure 4. Left testicle appears normal.
Figure 5. Normal appearing left testicle with intact vascular flow on Color Doppler.
Due to concern for testicular rupture, Urology was consulted. Urology took the patient to the operating room emergently for a scrotal exploration. The operative note described hematoma within the right scrotum and a disrupted tunica albuginea. The right testicle was shattered and it was determined that the testicle could not be preserved, prompting them to perform a right orchiectomy. The penrose drain was removed the next day and the patient was discharged with outpatient Urology follow-up.
Discussion
The typical appearance of a testicle on ultrasound involves a homogeneous testis with a surrounding echogenic outline representing the tunica albuginea, the dense fibrous middle layer encasing the testicle.Testicular rupture occurs secondary to testicular trauma, usually from blunt rather than penetrating injuries, or iatrogenic injury during scrotal surgeries.1 Scrotal ecchymosis, edema, and tenderness are common presenting physical exam findings. The edema often prevents the examiner from being able to palpate the margins of the testicle itself. Scrotal ultrasound often shows an enlarged, heterogeneous testis, surrounding hematocele, disrupted tunica albuginea and loss of Doppler flow to testicular parenchyma.
Heterogeneity of the testicle is concerning for tissue injury. Hypoechoic areas in the testis on ultrasound in the setting of testicular trauma suggest areas of tissue ischemia.4 The edema caused by testicular trauma increases intra-testicular pressure, potentially obstructing venous outflow and resulting in tissue infarction. In this case, the testicle was heterogeneous, concerning for tissue ischemia, although color Doppler did show blood flow to most of the testicular parenchyma (Figure 3).
A hematocele is a collection of blood within the scrotum surrounding the testis. A fresh hematocele can appear anechoic and some turbulence can be noted within. As time progresses, the hematocele will present with increased echogenicity and septae, differentiating it from an anechoic hydrocele.2 Although not appreciated in this case, testicular rupture may also result in the extrusion of seminiferous tubules. A scrotal hematocele will lack the arterial blood flow that will be seen on Doppler ultrasound for seminiferous tubules.3 In this case, a large scrotal echogenic hematocele was appreciated with formation of septae surrounding the testis. No extrusion of seminiferous tubules could be seen as there was no doppler flow in the surrounding structure.
Unlike a testicular fracture, which preserves the contour of the testis, the trauma in testicular rupture causes disruption of the tunica albuginea.5 As in our case, the margins and contour of the right testis were obscured, as shown by the lack of continuity in the echogenic line surrounding it, correlating to a disrupted tunica albuginea. Disruption of the tunica albuginea may in turn result in extrusion of contents from within the testis into the scrotum. Disruption of the tunica albuginea typically impedes vascular flow to the testicle as the innermost vascular layer forming the capsule around the testicle, the tunica vasculosa, is also usually involved. Therefore, Doppler flow usually shows loss of vascular flow to parts or the entirety of the testicular parenchyma.5
Testicular rupture does require emergent evaluation by Urology and disruption of the tunica albuginea necessitates surgical exploration. In some cases, the testicle may be preserved, especially in minor trauma or in early surgical exploration, but typically the extent of damage and ischemia requires orchiectomy.6
Conclusion
Point-of-care ultrasound proves useful in the evaluation of testicular trauma. As illustrated by this case, ultrasound may help characterize testicular injury and help direct management.
References
- Lucky, M., et al. British Association of Urological Surgeons (BAUS) consensus document for the management of male genital emergencies ‐ testicular trauma. BJU International. 2018. 121(6):840.
- Sommers, D., Winter, T. Ultrasonography evaluation of scrotal masses. Radiol. Clin. North Am. 2014. 52(6):1265-81.
- Blok, D., Flannigan, M., Jones, J. Testicular Rupture Following Blunt Scrotal Trauma. Case Rep. Emerg. Med. 2019. 18.
- Bhatt, S., Dogra, V. Role of US in testicular and scrotal trauma. Radiographics. 2008. 28(6):1617-29.
- Rao, M., Arjun, K. Sonography of scrotal trauma. Indian J. Radiol. Imaging. 2012. 22(4):293-7.
- Cass, A., Luxenberg, M. Testicular injuries. Urology. 1991. 27(6):528-30.
POCUS Use for Diagnosing Retained Products of Conception
Douglas Barber M.D. and Zachary Boivin M.D.
Case
A 31-year-old woman, G5P3 presented 7 days after a medical abortion with lower abdominal pain and increased vaginal bleeding. She reported soaking through one menstrual pad every two hours and passing large clots. She had 8 out of 10 abdominal pain described as “crampy” despite NSAID and acetaminophen use, ultimately requiring parenteral opioids. She endorsed lightheadedness when standing and was hemodynamically stable. Her hemoglobin, previously normal, was 9.5 × 103/µL. A transabdominal point-of-care ultrasound (POCUS) showed thickened endometrium measuring 1.45 cm (Figure 1; Video 1). Color Doppler showed blood flow within the echogenic intrauterine contents (Video 2).
Figure 1: Transabdominal sagittal image of the pelvis showing uterus with thickened endometrium measuring 1.45 cm.
Video 1: Transabdominal sagittal video clip of the pelvis showing echogenic intrauterine contents. The vaginal vault is inferior and posterior to the uterus and is distended with both echogenic and hypoechoic material.
Video 2: Transabdominal sagittal video clip of duplex ultrasound demonstrating blood flow to the intrauterine contents on Color Doppler.
The patient subsequently underwent a radiology-performed transvaginal ultrasound confirming intrauterine debris with internal vascularity prompting obstetrics and gynecology (OBGYN) consultation. The diagnosis of retained products of conception (RPOC) was made based on ultrasound and OBGYN evaluation. Given the degree of vaginal bleeding, she underwent dilation and evacuation with pathology notable for necrotic immature chorionic villi consistent with RPOC. She was discharged the next day on NSAIDs with trivial vaginal bleeding.
Discussion
Vaginal bleeding and lower abdominal pain are common presentations to the Emergency Department. Emergency physicians must distinguish expected post-abortion and post-partum bleeding from retained products of conception, which may benefit from medical or surgical management and OBGYN consultation. RPOC can be diagnosed in the setting of miscarriage, medical abortion, and after a birth. Radiology-performed transvaginal ultrasound is commonly performed to evaluate for RPOC, with literature suggesting that a normal-appearing uterus effectively rules out RPOC.1 Endometrial thickness > 10 mm appears relatively sensitive for the diagnosis, but there are also studies showing > 15 mm is more specific.2-4 The gold-standard for diagnosis is surgical pathology, and ultimately the management decision depends on the clinical situation and patient preference, as other options include medical management with misoprostol or expectant management.
This case demonstrates that RPOC can be identified transabdominally via POCUS, while radiology-performed ultrasound often uses the transvaginal approach. While there are limitations, such as reduced image quality especially in the setting of an under-distended bladder, the transabdominal approach is more feasible in the Emergency Department as it is less invasive, less time consuming, and more familiar to the emergency physician. It should be noted that while presence of blood flow to intrauterine contents raises suspicion for RPOC, absence of color Doppler flow does not rule the diagnosis out. Other diagnoses, such as hematometra, which will not have color Doppler flow, is managed similarly to RPOC.1 More research is needed to determine the diagnostic performance of POCUS for RPOC, but this case shows how ultrasound can be used to facilitate an early diagnosis of RPOC.
References
- Durfee, S.M., Frates, M.C., Luong, A. and Benson, C.B. (2005), The Sonographic and Color Doppler Features of Retained Products of Conception. Journal of Ultrasound in Medicine, 24: 1181-1186. https://doi.org/10.7863/jum.2005.24.9.1181
- Sellmyer M, Desser T, Maturen K, Jeffrey R, Kamaya A. Physiologic, Histologic, and Imaging Features of Retained Products of Conception. Radiographics. 2013;33(3):781-96. doi:10.1148/rg.333125177
- Tohma YA, Dilbaz B, Evliyaoğlu Ö, Çoşkun B, Çolak E, Dilbaz S. Is ultrasonographic evaluation essential for diagnosis of retained products of conception after surgical abortion? J Obstet Gynaecol Res. 2016;42(5):489-95. https://doi.org/10.1111/jog.12944.
- Hamel CC, van Wessel S, Carnegy A, Coppus S, Snijders M, Clark J, et al. Diagnostic criteria for retained products of conception-A scoping review. Acta Obstet Gynecol Scand. 2021;100(12):2135-43. https://doi.org/10.1111/aogs.14229
POCUS Saves
Free Fluid in the Setting of Acute Abdominal Pain
CPT Kaegan Williams, DSc, PA-C; CPT Summer Villa, MD; and Melissa Myers, MD FAAEM
Case
A 27-year-old otherwise healthy female presented to the emergency department complaining of abdominal pain starting approximately 90 minutes prior to arrival. The patient described the pain as sharp, constant, and non-radiating. The patient noted that the pain began during intercourse. She denied a history of STIs, dysuria, hematuria, and vaginal bleeding; however, she admitted to nausea. Her last menstrual cycle ended two weeks ago.
On physical examination, the patient appeared uncomfortable but cooperative. Vital signs were within normal limits. Abdominal examination was positive for tenderness in the lower quadrants, more prominent on the right side, and the patient also had mild rebound tenderness.
Bedside POCUS was conducted and displayed positive free fluid in the right upper quadrant (Figure 1) and pelvic region both sagittal (Figure 2) and transverse (Figure 3) views.
Figure 1
Figure 2
Figure 3
Due to the positive bedside ultrasound coordination occurred with radiology to perform a CT of the abdomen and pelvis with contrast before the lab-work, including pregnancy test, resulted. Radiology called a ruptured right hemorrhagic cyst with moderate blood products in the pouch of Douglas and no visible fetus (Figures 4, 5).
Figure 4
Figure 5
Labs were remarkable for leukocytosis to 17.06 with a left shift consistent with the clinical picture, a negative pregnancy test, normal hemoglobin, hematocrit, and urinalysis inconsistent with UTI. PT received 1G IV Acetaminophen and 15mg of IV Ketorolac. Upon reassessment, the patient had significant improvement in her symptoms with complete resolution of her discomfort. The treatment team informed the patient of the findings of a ruptured hemorrhagic cyst. The treatment team discussed different treatment options, and through shared decision making, the patient felt comfortable going home with watchful waiting. She expressed understanding with return precautions and felt able to be seen by her PCM within the next week. She was seen by her PCM who placed a referral to gynecology for evaluation of the cyst, no issues or concerns were noted during that encounter.
Discussion
Hemorrhagic ovarian cysts occur when a blood vessel in the wall of a cyst ruptures and blood enters the cyst. Approximately 90% of these cysts resolve spontaneously, but in some cases, the cyst can rupture. Typically, simple and hemorrhagic cysts less than 5 cm resolve in one to two menstrual cycles. Ruptured hemorrhagic cysts can present similarly to ectopic pregnancy, tubo-ovarian abscess, or ovarian torsion.1,2 A rupture can lead to the spillage of cystic fluid and blood into the peritoneal cavity, leading to symptoms such as acute abdominal pain, often localized to the side of the ruptured cyst. The pain can be sharp and sudden and may present with nausea or vomiting.
In the Emergency Department, point of care ultrasound (POCUS) allows for rapid and effective diagnosis of ruptured hemorrhagic cysts. If transvaginal ultrasound is available, the ovary may be directly visualized. In an acute rupture, the clot may have a similar echogenicity to the surrounding ovary with asymmetrical ovarian enlargement as the only sign of the ruptured cyst.3 As a hemorrhagic cyst ages, it will develop mixed echogenicity, reflecting the formation of clots and active bleeding. The hemorrhagic cyst may have a “spider’s web” or “lace net” appearance.2-3
Many Emergency Physicians do not have access to transvaginal ultrasound at the point of care, and in this case, the Focused Assessment in Trauma (FAST) exam may be used for diagnosis in the appropriate clinical setting. The presence of free fluid in the right upper quadrant (RUQ) on the FAST in the setting of a bleeding hemorrhagic cyst indicates a clinically significant bleed. A minimum of 225mL, and more commonly at least 400mL of fluid is needed for the fluid to be visible in the RUQ.4 Physicians should bear in mind that up to 40% of women will have physiologic free fluid in the rectovaginal pouch during ovulation.2 However, a large quantity of free fluid in the pelvis or detection of free fluid in the upper quadrants is concerning for a clinically significant, actively bleeding hemorrhagic cyst.
The management of ruptured hemorrhagic cysts is primarily conservative in hemodynamically stable patients, consisting of pain control, hydration, and monitoring. Surgical intervention is reserved for cases where there is significant internal bleeding, ongoing pain despite conservative management, or when the diagnosis is uncertain and other conditions, such as ectopic pregnancy or appendicitis, cannot be ruled out. Complications of ruptured hemorrhagic cysts include hemoperitoneum, hypovolemic shock, and peritonitis. These complications underline the importance of early diagnosis and appropriate management.1,2
Conclusion
Hemorrhagic ovarian cysts can lead to severe complications such as cyst rupture and hemoperitoneum. Early detection and management can significantly improve patient outcomes. Using POCUS in emergency settings can expedite diagnosis and management, reducing the risk of severe complications.
References
- McWilliams, Grant D. E., et al. “Gynecologic Emergencies.” Surgical Clinics of North America, vol. 88, no. 2, 2008, pp. 265–83, https://doi.org/10.1016/j.suc.2007.12.007.
- Bottomley C, Bourne T. Diagnosis and management of ovarian cyst accidents. Best Practice & Research Clinical Obstetrics & Gynaecology. 2009;23(5):711-724. doi:10.1016/j.bpobgyn.2009.02.001
- Naffaa, Lena, et al. “Imaging of acute pelvic pain in girls: ovarian torsion and beyond☆.” Current problems in diagnostic radiology 46.4 (2017): 317-329.
- Branney, Scott W., et al. “Quantitative sensitivity of ultrasound in detecting free intraperitoneal fluid.” Journal of Trauma and Acute Care Surgery 39.2 (1995): 375-380.
The Use of POCUS to Diagnose Ruptured Epidermoid Inclusion Cyst
Zachary Ginsberg, MD and Gabriela Rivera-Camacho, MD
Case
A 72-year-old female with a history of type II diabetes mellitus and hypothyroidism presented to the Emergency Department with a painful mass on the suboccipital region over the past week. She attempted to squeeze the mass with subsequent production of pus and blood.
She presented with a fever of 38.1°C, no leukocytosis, and normal vital signs. The mass was about 5 x 2 cm in size on clinical examination. On point-of-care ultrasound, the mass appeared heterogeneous without loculations (Figure 1). There were several linear and regularly-shaped small anechoic structures, less consistent with a drainable fluid collection. The surrounding skin was thickened and cobblestoning was appreciated (Figure 2).
Figure 1: Lobulations, tissue heterogeneity, and vascular-like structures seen within the mass.
Figure 2: The tissue surrounding the mass showed skin thickening and cobblestoning, suggestive of cellulitis.
Due to concern for possible tumor, CT with IV contrast was obtained which revealed a large suboccipital, cutaneous heterogeneous area with small circumscribed regions with peripheral enhancement in the subcutaneous tissue, concerning for cellulitis/abscess or possible malignancy. General surgery was consulted for possible deep abscess with surrounding cellulitis. General surgery recommended a surgical oncology consult to rule out malignancy.
During the admission, a punch biopsy was performed and it revealed a ruptured epidermoid inclusion cyst. She was discharged from the hospital on day 4 on a 7-day course of trimethoprim/sulfamethoxazole to treat the superimposed skin infection. On outpatient follow-up, the patient denied recurrence of the symptoms and she deferred surgical excision to prevent reaccumulation.
Discussion
Epidermoid inclusion cysts are the most common of cutaneous cysts, which arise from either a plugged hair follicle or when the follicular epithelium is implanted into the dermis layer.1 They usually appear as fluctuant nodules and can have a central punctum. Inflammation can lead to cyst rupture. When ruptured, the cysts tend to be painful and cause extrusion of the contents within the cyst into the surrounding areas, serving as a possible nidus for infection.1 Additionally, keratin contents lead to granulation tissue formation.2
Unruptured epidermoid cysts tend to appear hypoechoic with clear boundaries, halo, and no Doppler color flow within.2 The majority are round in shape, but may be lobulated or tubular too.2 Smaller ones are usually homogeneous, but may develop some heterogeneity with increased size due to fat, pus, mucoid, or calcification.2 When ruptured, the majority have a lobulated shape, less defined borders, lack of halo, and increased vascularity.3 Twinkle artifact is also reported as a common finding in ruptured epidermoid inclusion cysts, with studies suggesting favorable specificity for superficial masses requiring surgical excision.4
In this patient’s bedside ultrasound, a lobulated mass with poorly defined borders, suspected vascularity, and tissue heterogeneity was appreciable (Figure 3).
These findings supported the diagnosis of ruptured epidermoid inclusion cyst. In retrospect, color Doppler should have also been obtained to assess for color flow signal and confirm vascularity, and possible twinkle artifact.
Conclusion
Bedside ultrasound serves as a useful diagnostic tool for cutaneous masses. This case demonstrates the ability to use ultrasound to characterize a mass prior to management decisions, such as avoiding an unnecessary incision and drainage in a mass that does not appear like fluid collection such as an abscess on ultrasound. The use of color doppler aids in identifying other abscess mimics such as pseudoaneurysms, lymph nodes, Baker’s cyst, or epidermoid inclusion cyst as noted in this patient. Typically, malignant lesions tend to be hypervascular, in which an increased color doppler signal would be expected suggesting hyperemia. Ultrasound also helps distinguish between ruptured and unruptured epidermoid inclusion cysts.
References
- Weir, CB, St. Hilaire, NJ. Epidermoid Inclusion Cyst. StatPearls. 2023.
- Lee, HS, et al. Relationship between sonographic and pathologic findings in epidermal inclusion cysts. J. Clin. Ultrasound. 2001; 29(7):374-383.
- Yuan, WH, et al. Differences in sonographic features of ruptured and unruptured epidermal cysts. J. Ultrasound Med. 2012; 31(2):265-272.
- Clarke, R, et al. Twinkle artifact in the ultrasound diagnosis of superficial epidermoid cysts. Ultrasound. 2016; 24(3):147-153.
Procedure Section
Identification of Costal Cartilage Fractures with Ultrasound in the Emergency Department
Kristen Purcell, DO; Jamie Baydoun, MD FAAEM; and Kristina Domanski, MD
Introduction
Costal cartilage is integral for the structural stability of the rib cage. Rib fractures are a common sequela of blunt thoracic trauma and occasionally missed on standard radiography leading to delay in diagnosis, poor pain control, and the potential for pulmonary complications. Costal cartilage fractures are thought to be considerably underreported because of the poor sensitivity of standard imaging studies utilized during evaluation of thoracic trauma.1 Complications of costal cartilage fractures are similar to rib fractures including pain that persists for several months after the initial trauma, pulmonary complications from inadequate pulmonary hygiene, and structural deformities such as nonunion and rib cage asymmetry.1,2 In this case report, we identify the utilization of ultrasound for evaluation of isolated costal cartilage fractures in the Emergency Department (ED).
Case
A 28 year old otherwise healthy male presented to the ED complaining of left lower chest wall pain after he felt a “pop” while twisting his torso during Jiu Jitsu practice one week prior. Following the injury, he noted that the lower part of his chest wall “felt like it was popping in and out of place” during daily activities requiring use of core muscles. On the day of the injury he presented to an urgent care where a chest x-ray and rib series were obtained which were interpreted as unremarkable. The patient presented to our facility three days after his initial injury for concern of ongoing pain and the uncomfortable “popping” sensation he felt at the left lower chest wall with movement. Physical exam was remarkable for tenderness over the mentioned area with no notable deformity, however, a palpable “pop” was noted on flexion of the torso over this region. The ED physician utilized POCUS with the linear ultrasound transducer to evaluate the area of pain and tenderness to the chest. A mildly displaced step off deformity of the left 7th costal cartilage was identified, which was consistent with a fracture (figure 1). An image of the contralateral side was obtained for comparison which demonstrated intact costal cartilage (figure 2). The patient was provided prescriptions for lidocaine patches, cyclobenzaprine, and ibuprofen with instructions on pulmonary hygiene. On telephone follow up at one and three months following the patient’s visit, he reported adequate pain control with return to his baseline activities.
Figure 1: Longitudinal view of the left 7th costal cartilage with disruption of the anterior echogenic margin consistent with a fracture.
Figure 2: Longitudinal view of the right 7th costal cartilage showing intact anterior echogenic margin for comparison.
Discussion
Identification of costal cartilage rib fractures on ultrasound is accomplished by examining the location of discomfort and finding evidence of cortical disruption on the hyperechoic anterior surface of the cartilage.3,4 Costal cartilage fractures are typically seen in conjunction with rib fractures following blunt trauma. Isolated costal cartilage fractures have been reported in sporting activities due to blunt trauma or twisting motions as well as from violent episodes of vomiting or coughing.5,6 While isolated costal cartilage fractures are typically benign, they can lead to significant pain resulting in respiratory complications and limitations in performing daily activities. Identification of costal cartilage fractures is important, as proper diagnosis allows for adequate pain control and pulmonary hygiene similar to treatment for rib fractures. Additionally, if there is structural compromise of the thoracic wall secondary to these fractures, operative repair may be indicated.7,8 Prior studies have noted poor sensitivity of x-ray and computed tomography in comparison to ultrasound for evaluation of costal cartilage fractures in addition to risk of radiation exposure.3,9 MRI is another modality that has been utilized effectively in evaluating for costal cartilage fractures, however, given the time and financial cost of this study it may not be as favorable for rapid diagnosis in the ED.10 Ultrasound has become a standard tool in the emergency department and has been previously shown to be beneficial in identifying rib and costal cartilage fractures missed on conventional imaging. This case further demonstrates the ability for emergency medicine physicians to utilize the ultrasound in diagnosis of costal cartilage fractures.
Conclusion
Diagnosis of costal cartilage fractures can be easily performed by the emergency medicine physician by using linear ultrasound probe. Bedside ultrasound allows for prompt recognition, decreased radiation exposure, initiation of appropriate pain control and pulmonary hygiene as well as surgical referral as indicated for structural instability.
References
- Meteb M, Abou Shaar B, El-Karim GA, Almalki Y. Costal cartilage fracture: A commonly missed thoracic injury in trauma patients. Radiol Case Rep. 2021 Nov 1;17(1):95-98. doi: 10.1016/j.radcr.2021.10.008. Erratum in: Radiol Case Rep. 2023 Mar;18(3):1387-1388. PMID: 34765069; PMCID: PMC8571527.
- Nummela MT, Pyhältö TT, Bensch FV, Heinänen MT, Koskinen SK. Costal cartilage fractures in blunt polytrauma patients – a prospective clinical and radiological follow-up study. Emerg Radiol. 2022 Oct;29(5):845-854. doi: 10.1007/s10140-022-02066-w. Epub 2022 Jun 4. PMID: 35661281; PMCID: PMC9458556.
- Turk, F., Kurt, A.B. & Saglam, S. Evaluation by ultrasound of traumatic rib fractures missed by radiography. Emerg Radiol 17, 473–477 (2010). https://doi.org/10.1007/s10140-010-0892-9
- Malghem J, Vande Berg B, Lecouvet F, Maldague B. Costal cartilage fractures as revealed on CT and sonography. AJR Am J Roentgenol. 2001 Feb;176(2):429-32. doi: 10.2214/ajr.176.2.1760429. PMID: 11159088.
- Daniels SP, Kazam JJ, Yao KV, Xu HS, Green DB. Cough-induced costal cartilage fracture. Clin Imaging. 2019 May-Jun;55:161-164. doi: 10.1016/j.clinimag.2019.03.007. Epub 2019 Mar 13. PMID: 30897383.
- Drakonaki E, Karageorgiou I, Kokkinakis S, Maliotis N, Spyridaki R, Symvoulakis EK. Vomiting-induced costal cartilage fracture: a case report. Med Ultrason. 2022 Feb 16;24(1):117-119. doi: 10.11152/mu-2677. Epub 2020 Oct 20. PMID: 33626124.
- Li Y, Zhao Y, Yang Y, Wu W, Guo X, Zhao T. Surgical treatment of costal cartilage fractures with titanium plate internal fixation. J Cardiothorac Surg. 2022 Mar 28;17(1):57. doi: 10.1186/s13019-022-01801-1. PMID: 35346289; PMCID: PMC8961934.
- Sollender GE, White TW, Pieracci FM. Fracture of the Costal Cartilage: Presentation, Diagnosis, and Management. Ann Thorac Surg. 2019 Apr;107(4):e267-e268. doi: 10.1016/j.athoracsur.2018.08.076. Epub 2018 Oct 22. PMID: 30359588.
- Lee, W.S., Kim, Y.H., Chee, H.K. et al. Ultrasonographic evaluation of costal cartilage fractures unnoticed by the conventional radiographic study and multidetector computed tomography. Eur J Trauma Emerg Surg 38, 37–42 (2012). https://doi.org/10.1007/s00068-011-0117-2
- Subhas N, Kline MJ, Moskal MJ, White LM, Recht MP. MRI evaluation of costal cartilage injuries. AJR Am J Roentgenol. 2008 Jul;191(1):129-32. doi: 10.2214/AJR.07.3396. PMID: 18562735.
Performing Ultrasound-guided Intercostal Nerve Blocks in Patients with Acute Rib Fracture Pain
Bushra Hussein, MD and Rebecca Theophanous, MD, MHSc
Case Presentation
An 85-year-old-male arrives to the emergency department via ambulance with chest wall pain after a mechanical fall from standing. He has a large contusion on his right posterior chest wall and is taking shallow quick breaths from the pain. His oxygen saturation is 91% on room air, so the patient is placed on two liters of supplemental oxygen. Chest x-ray shows multiple right-sided lower thoracic rib fractures and chest computed tomography scans reveals a right-sided apical pneumothorax. How can you help treat the patient’s pain without contributing to delirium and to prevent worsening of his respiratory symptoms and/or complications such as pneumonia?
Ultrasound-guided nerve blocks are a useful bedside tool for achieving more sustained pain control in settings of acute pain or trauma. The Intercostal Nerve Block (ICNB) is a relatively safe and easy-to-perform block that can improve pain control, potentially reduce delirium from medication adverse effects such as opioids and can lessen or prevent complications in patients with acute rib fractures, especially in the elderly population.1-2
Indications
ICNB is an appropriate method of regional anesthesia in patients with acute rib fracture pain, post-surgical pain after chest wall or upper abdominal surgery, or other causes of severe thoracic musculoskeletal pain. It is best used at the T7-T11 level due to the posterior approach because the scapula and rhomboid muscles are obstructive at higher levels. Thus, paravertebral or thoracic epidural blocks are likely better options for upper levels. ICNB is ideal for one-sided rib pain (either right or left) to avoid the very minimal possibility of bilateral pneumothorax and to avoid local anesthetic systemic toxicity given the higher amounts of anesthetic required. ICNB cannot be used for visceral pain from intra-abdominal organs.1-2
Contraindications
ICNB is contraindicated in patients on active anticoagulation, with severe bleeding risk, or with local infection overlying the potential area of needle insertion. Furthermore, it should only be performed after proper training and expertise, and with the appropriate equipment and cardiopulmonary monitoring in place. 1-3
Thoracic Anatomy
The neurovascular bundle is located inferior to each rib at the subcostal groove. The intercostal nerve lies caudal or inferior to the intercostal artery than vein. ICNB blocks the ipsilateral sensory and motor fibers of the ICNs. The ICN can be blocked anywhere proximal to the midaxillary line, where the lateral cutaneous branch takes off. The nerves typically can have additional innervation to the level above and below the respective rib space, and some may also enter the paravertebral space. 1-2
Suggested Equipment
- Ultrasound machine with high-frequency linear transducer (8-13 MHz)
- Antimicrobial skin cleanser
- Sterile and Personal Protective Equipment including gloves, gown, drapes, probe cover, sterile gel, etc.
- Marking pen
- 20G or 22G needle with 1.5inch length (4-5cm)
- Local anesthetic (e.g. lidocaine, bupivacaine, ropivacaine)
- Syringes (e.g. 3ml, 10ml, 20ml)
- Optional extension tubing between the needle and syringe if performing with an assistant
- 2×2 gauze and band-aids
- Cardiopulmonary monitors and resuscitative equipment (available in case of complication)
- Lipid emulsion 20% (available at bedside)
Step-by-Step Approach and Technique
a) Optimize patient positioning (Figure 1): The patient should be sitting upright with their arms hugging a pillow across a table and leaning slightly forward to retract the scapula. The patient should be attached to cardiopulmonary monitoring throughout the procedure with supplemental oxygen and intravenous lipid emulsion therapy available at the bedside. The skin should be cleaned with antiseptic solution and proper sterile technique used throughout the procedure.2-3
Figure 1: Dermatomes
b) Mark landmarks (Figure 2): The inferior aspect of the pertinent 7th-11th ribs should be marked approximately 7cm away from the midline on the patient’s right or left back. The level of the pain or fracture should be marked, as well as the level above and below.
Figure 2: Intercostal Nerve Block Anatomy
c) Ultrasound probe position (Figure 3): To mark the ribs, the high-frequency linear transducer (8-13 Hz) should be oriented in a sagittal position to identify the external, internal, and innermost intercostal muscle layers between the two ribs, with the pleural space deep to these structures. Color doppler should be used to visualize the blood vessel vascular flow to avoid these structures.
Figure 3: Ultrasound Probe Position Landmarks
d) Needle insertion (Figure 4): The needle should be inserted with the bevel pointing up and directed cephalad at a 20-degree angle in a similar plane with the ribs. The needle should be advanced slowly in-plane under ultrasound-guidance 1mm at a time at the inferior edge of the rib under the costal groove until the needle touches the bony periosteum.
Figure 4: Needle Insertion
e) Local anesthetic injection (Figure 5): Aspirate to ensure no blood return then inject a small amount of anesthetic at the periosteum. Then retract the needle and reapproach another 2-3mm inferior to the rib until a “pop” is felt when penetrating the fascia near the internal intercostal muscle. Aspirate then inject 3-5mL anesthetic between the innermost intercostal muscle and the internal intercostal muscle layers, then retract the needle (Figure 6).
Figure 5: Local Anesthetic Injection
Figure 6: Needle Path
f) Repeat the process at the rib level above and again at the level below, injecting 3-5mL at each level to maximize anesthetic effect.2-3
Helpful Tip: A two-person technique can be used so that one person is introducing the needle under ultrasound-guidance while the other person is aspirating and injecting the anesthetic (Figure 6).
Anesthetic Medication Properties
Local anesthetic medication options, dosing, and duration of action are listed in the following table. Typically, 3-5mL should be injected at the level above, at, and below the pain, with a dose of about 0.1 to 0.15 ml/kg per intercostal space. The addition of epinephrine to bupivacaine or ropivacaine can increase the maximum injected dose by about 30% due to slower systemic absorption. Typical duration of action is 12 ± 6 hours.5-6
| Anesthetic Medication Name | Concentration | Maximum Dosing | Duration of action |
| Lidocaine | 1% | 5 mg/kg/injection | 1-2 hours |
| Lidocaine w/ Epinephrine | 1-2% | 7 mg/kg/injection | 2-6 hours |
| Bupivacaine | 0.25-0.5% | 2-3 mg/kg/injection | 2-4 hours |
| Ropivacaine | 0.5% | 4 mg/kg/injection | 2-4 hours |
Potential Complications
Arterial puncture, bleeding, infection, hemo/pneumothorax, and muscle laceration or injury are possible although rare.5-6
Local anesthetic systemic toxicity (LAST) is a possible complication that can be avoided with appropriate dosing and should be recognized early. Symptoms can include dizziness or light-headedness, tongue paresthesia, circumoral numbness, palpitations or tachycardia, agitation, nervousness, confusion, tinnitus, muscle twitches, seizures, and cardiac/neurological toxicity.5-6
Key Points
- ICNB is best performed from the posterior approach, at levels T7-T11 due to the scapula and rhomboid muscles. Higher thoracic levels or bilateral rib fractures can be blocked using alternative techniques such as thoracic paravertebral or epidural nerve blocks.
- The patient should be appropriately positioned with the inferior aspect of each rib marked and ultrasound-guidance used throughout to minimize risk of complications.
- The ideal entry angle is 20 degrees cephalad with needle insertion inferior to the rib at the subcostal groove, approximately 7cm lateral to the midline.
- Aspiration prior to anesthetic injection is critical, with slow advancement in-plane and deposition of 3-5ml of anesthetic at the appropriate rib level, plus the level above and level below for best analgesia effects due to collateral innervation.
- The total amount of anesthetic to be injected should be calculated prior to the procedure start to prevent anesthetic toxicity. The patient should be attached to cardiopulmonary monitoring devices at all times, and intravenous lipid emulsion 20% therapy should be available at the bedside.
Patient Case Resolution
The patient and his family consented to a regional nerve block for improved pain management from his acute rib fractures. After stabilization with supplemental oxygen, ultrasound-guidance was used to perform an intercostal nerve block, and the patient was admitted to the intensive care unit for further treatment of his traumatic injuries.
References
- Ho AM, Buck R, Latmore M, et al. Intercostal Nerve Block – Landmarks and Nerve Stimulator Technique. Nysora 2023. https://www.nysora.com/topics/regional-anesthesia-for-specific-surgical-procedures/thorax/intercostal-nerve-block/
- Lopez-Rincon RM, Kumar V. Ultrasound-Guided Intercostal Nerve Block. [Updated 2022 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555900/
- Gray AT. Chapter 54 – Intercostal Nerve Block. Atlas of Ultrasound-Guided Regional Anesthesia (Third Edition). Elsevier. 2019: 233-238. ISBN 9780323509510. https://doi.org/10.1016/B978-0-323-50951-0.00054-2. https://www.sciencedirect.com/science/article/pii/B9780323509510000542#f0010.
- El-Boghdadly, K. and Wiles, M.D. (2019), Regional anaesthesia for rib fractures: too many choices, too little evidence. Anaesthesia, 74: 564-568. https://doi.org/10.1111/anae.14634. https://associationofanaesthetists-publications.onlinelibrary.wiley.com/doi/10.1111/anae.14634
- Collins JB, Song J, Mahabir RC. Onset and duration of intradermal mixtures of bupivacaine and lidocaine with epinephrine. Can J Plast Surg. 2013 Spring;21(1):51-3. doi: 10.1177/229255031302100112. PMID: 24431939; PMCID: PMC3891109. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3891109/ ~:text=Lidocaine%20is%20known%20to%20have,epinephrine%20(6%2C7).6. Lagan G, McLure HA: Review of local anaesthetic agents. Curr Anaesth Crit Care 2004;15:247–254.
EUS-AAEM Poster Competition Winners from AAEM24
1st Place: One Twin is Not Like the Other! – Kelsey Sklar, MD MPH
2nd Place (tied):
- Not Your Typical Elbow Bump – Samuel A. Shulimson, DO
- Fetal Pericardial Effusion in a Bicornuate Uterus: Think Unicorns, Not Zebras – Amna Jamshad, DO
3rd Place: An Unexpected Inguinal Guest – Jonathan Knisley, MD JD
